Dubai: Scientists around the world had been hard at work to find antivirals specific to SARS-CoV-2, the virus that causes COVID-19.
Several drugs — such as chloroquine, arbidol and remdesivir and various other drug combinations — have undergone clinical studies to test their efficacy and safety in combating the coronavirus.
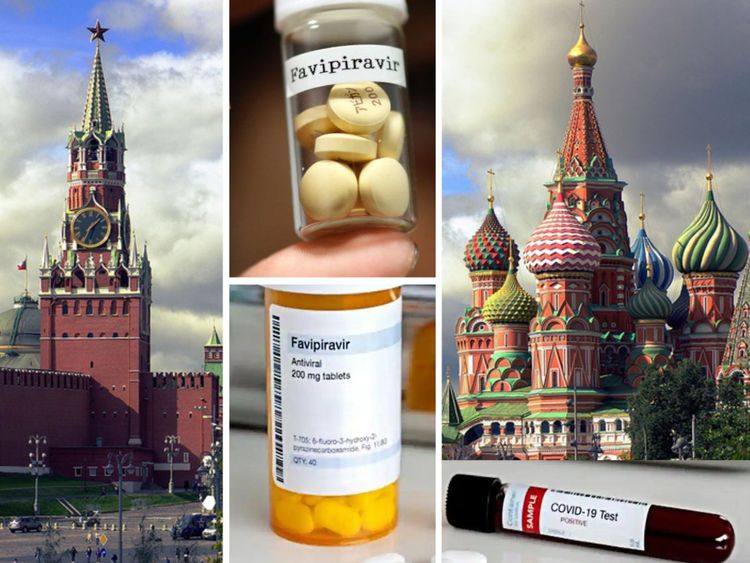
Now, Russia has licensed the antiviral drug Avifavir against coronavirus. Russian health authorities have given a green light to a clinical trials.
Q: What was it meant for?
Avifavir is an anti-influenza medication in Japan approved in 2014.
It has been repurposed to fight COVID-19. Preliminary trials are said to have shown that Avifavir could shorten recovery times for patients with the coronavirus.
On May 30, 2020 (Saturday) Russia’s Ministry of Health approved Avifavir for emergency use to treat COVID-19, after “encouraging” results from clinical trials of the generic version (Favipiravir) in several countries, including China and Japan. It is Russia’s first COVID-19 drug.
Q: What is it based on?
Avifavir is based on Favipiravir (generic name), and originally sold in Japan under the brand name Avigan. It was developed by Japan’s Fujifilm Pharmaceuticals as an antiviral medication to treat influenza.
Favipiravir is a pyrazinecarboxamide derivative, and is also being studied to treat a number of other viral infections.
Q: How does it work?
Favipiravir works by blocking the ability of a virus to replicate inside a cell. Some doctors began trying Favipiravir to treat coronavirus patients early on, reasoning that its anti-viral properties would be applicable.